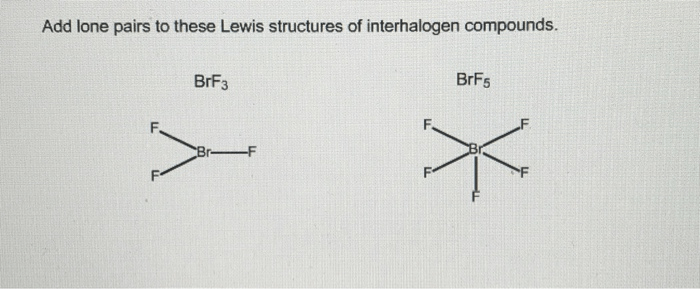
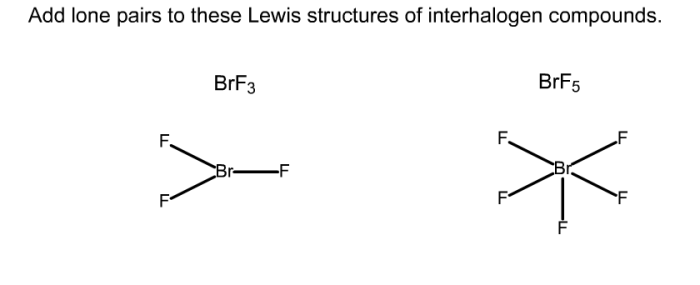
Add lone pairs to these lewis structures of interhalogen compounds – In the realm of chemistry, understanding the intricacies of interhalogen compounds is paramount. These fascinating substances exhibit unique properties that stem from their molecular structures. To delve into the depths of their electronic configurations, it is essential to add lone pairs to their Lewis structures, providing a comprehensive representation of their chemical bonding.
This guide will embark on a journey to elucidate the significance of adding lone pairs to Lewis structures of interhalogen compounds. We will explore the fundamental principles governing the number and placement of lone pairs, unravel the concept of resonance structures, and delve into specific examples to solidify our understanding.
Interhalogen Compounds: Add Lone Pairs To These Lewis Structures Of Interhalogen Compounds
Interhalogen compounds are molecules that contain two or more different halogens. They are typically formed by the direct reaction of two halogen elements, and they can have a variety of structures and properties.
Interhalogen compounds are often more reactive than the parent halogens, and they can exhibit a wide range of chemical behaviors. They are used in a variety of applications, including as disinfectants, bleaching agents, and oxidizing agents.
Lewis Structures of Interhalogen Compounds
The Lewis structure of an interhalogen compound shows the arrangement of the atoms and the bonding electrons in the molecule. The general guidelines for drawing Lewis structures of interhalogen compounds are as follows:
- The halogen atoms are arranged in a linear or bent chain.
- The central halogen atom is bonded to the terminal halogen atoms by single bonds.
- Each terminal halogen atom has three lone pairs of electrons.
For example, the Lewis structure of iodine monochloride (ICl) is:
Adding Lone Pairs
It is important to add lone pairs to Lewis structures of interhalogen compounds in order to satisfy the octet rule. The octet rule states that each atom in a molecule should have eight valence electrons. In the case of interhalogen compounds, the terminal halogen atoms have three lone pairs of electrons, which satisfies the octet rule.
The rules for determining the number and placement of lone pairs are as follows:
- Each terminal halogen atom has three lone pairs of electrons.
- The central halogen atom has one lone pair of electrons for each lone pair on the terminal halogen atoms.
For example, the Lewis structure of iodine monochloride (ICl) with lone pairs added is:
Resonance Structures, Add lone pairs to these lewis structures of interhalogen compounds
In some cases, it is possible to draw multiple Lewis structures for an interhalogen compound. These Lewis structures are called resonance structures. Resonance structures are different representations of the same molecule, and they have the same number of valence electrons.
For example, the Lewis structure of iodine monochloride (ICl) can be drawn as two resonance structures:
The two resonance structures of iodine monochloride have the same number of valence electrons, and they both satisfy the octet rule. The actual structure of iodine monochloride is a resonance hybrid of the two resonance structures.
FAQ Corner
What is the significance of adding lone pairs to Lewis structures?
Adding lone pairs to Lewis structures allows for a more accurate representation of the electron distribution within a molecule, providing insights into its chemical bonding and reactivity.
How do I determine the number of lone pairs to add?
The number of lone pairs is determined by the valence electrons of the atoms involved and the number of bonds formed. Lone pairs are added to satisfy the octet rule for each atom.
What is resonance in the context of interhalogen compounds?
Resonance occurs when multiple Lewis structures can be drawn for a molecule, each representing a different distribution of electrons. In interhalogen compounds, resonance structures can provide a more accurate depiction of the bonding and electron delocalization.