Mechanism nitration of methyl benzoate, a captivating chemical transformation, unfolds as a symphony of molecular interactions, offering a profound understanding of electrophilic aromatic substitution reactions. This intricate process unveils the nuances of regioselectivity, catalyst roles, and reaction conditions, laying the foundation for diverse applications in pharmaceuticals, dyes, and explosives.
The electrophilic aromatic substitution mechanism orchestrates a series of orchestrated steps, where the electrophile, a positively charged species, seeks a nucleophilic aromatic ring, initiating a dance of electron transfer and rearrangement. The regioselectivity of the reaction, influenced by electronic and steric factors, determines the precise orientation of the nitro group, shaping the final product’s properties and reactivity.
Mechanism of Nitration of Methyl Benzoate
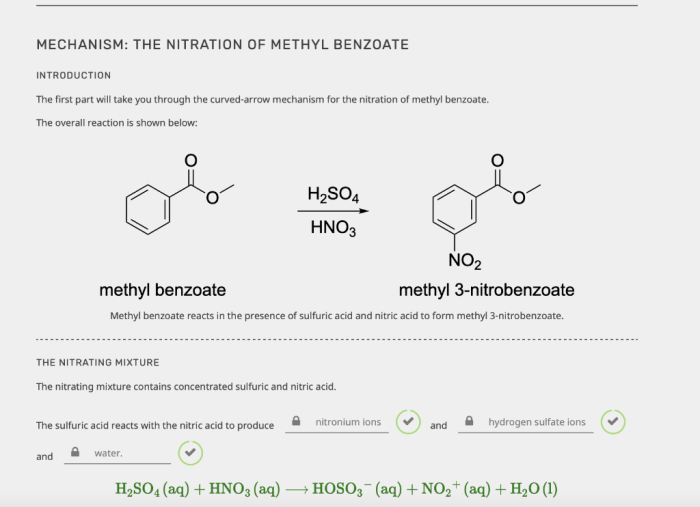
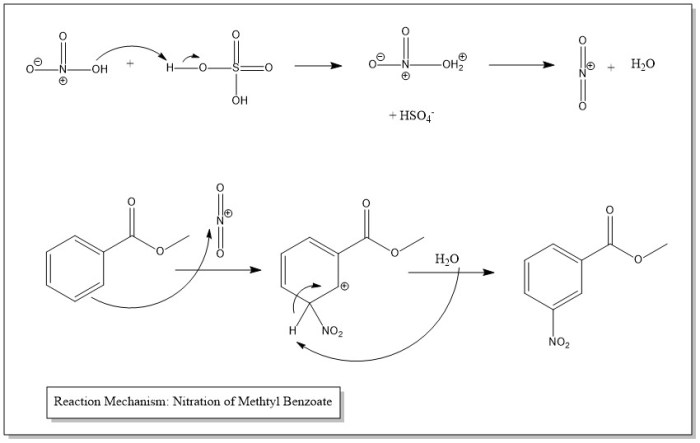
The nitration of methyl benzoate involves an electrophilic aromatic substitution reaction. The electrophile in this reaction is the nitronium ion (NO 2+), which is generated in situ from the reaction of nitric acid (HNO 3) and sulfuric acid (H 2SO 4). The reaction proceeds through the following steps:1.
-
-*Formation of the nitronium ion
Nitric acid and sulfuric acid react to form the nitronium ion, which is a powerful electrophile.
- 2.
- 3.
-*Electrophilic attack on the aromatic ring
The nitronium ion attacks the aromatic ring of methyl benzoate, forming a sigma complex.
-*Rearomatization
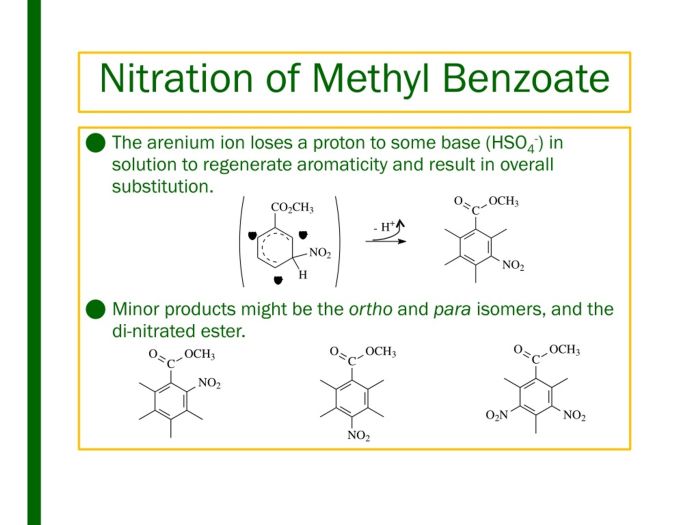
The sigma complex undergoes a rearrangement to form the nitrated product, methyl 3-nitrobenzoate, and regenerate the aromatic ring.
Regioselectivity of the Reaction
The nitration of methyl benzoate is regioselective, meaning that the nitro group is preferentially introduced at the meta position of the aromatic ring. This regioselectivity is due to the electron-withdrawing nature of the nitro group, which deactivates the ortho and para positions of the ring towards electrophilic attack.
Reagents and Reaction Conditions
The nitration of methyl benzoate requires the following reagents:
-
-*Methyl benzoate
The substrate for the reaction.
-*Nitric acid
The source of the nitronium ion.
-*Sulfuric acid
A catalyst that promotes the formation of the nitronium ion.
The reaction is typically carried out at room temperature in a mixture of nitric acid and sulfuric acid. The concentration of the reagents and the reaction time can be varied to control the yield and regioselectivity of the reaction.
Applications of Nitrated Methyl Benzoate, Mechanism nitration of methyl benzoate
Nitrated methyl benzoate is an important intermediate in the synthesis of a variety of products, including:
-
-*Pharmaceuticals
Nitrated methyl benzoate is used as a precursor to the synthesis of a number of drugs, such as aspirin and ibuprofen.
-*Dyes
Nitrated methyl benzoate is used as a starting material for the synthesis of a variety of dyes, such as methyl orange and congo red.
-*Explosives
Nitrated methyl benzoate is used as an explosive in a number of applications, such as mining and construction.
Alternative Methods for Nitrating Methyl Benzoate
In addition to the traditional method described above, there are a number of alternative methods for nitrating methyl benzoate. These methods include:
-
-*Nitration with mixed acid
Nitration can also be carried out using a mixture of nitric acid and acetic anhydride. This method is often used when the substrate is sensitive to the strong oxidizing conditions of nitric acid alone.
-*Nitration with nitronium salts
Nitration can also be carried out using nitronium salts, such as nitronium tetrafluoroborate (NO 2BF 4). These salts are more stable and easier to handle than nitric acid, making them a safer and more convenient option for nitration reactions.
Safety Considerations: Mechanism Nitration Of Methyl Benzoate
The nitration of methyl benzoate is a potentially hazardous reaction. The following safety precautions should be taken:
-
-*Wear appropriate personal protective equipment
Nitric acid and sulfuric acid are corrosive and can cause severe burns. Wear gloves, goggles, and a lab coat when handling these chemicals.
-*Work in a well-ventilated area
Nitric acid and sulfuric acid fumes are toxic and can cause respiratory irritation. Work in a well-ventilated area or use a fume hood.
-*Dispose of waste properly
Nitrated methyl benzoate and the reaction byproducts are hazardous waste. Dispose of these materials in accordance with local regulations.
Key Questions Answered
What factors influence the regioselectivity of the nitration of methyl benzoate?
Electronic and steric effects, such as the presence of electron-withdrawing or donating groups and the size of substituents, play a crucial role in determining the orientation of the nitro group.
What are the potential hazards associated with the nitration of methyl benzoate?
The reaction involves the use of concentrated nitric and sulfuric acids, which are corrosive and can cause severe burns. Additionally, the nitration products may be explosive, necessitating proper handling and storage.